
FSIS Ready-To-Eat Sampling Programs - Revision 1
CHAPTER I - GENERAL
- PURPOSE
- FSIS product sampling for Listeria monocytogenes (Lm) and Salmonella are important food safety verification activities that support FSIS' food safety and public health goals. This directive provides instructions to inspection program personnel (IPP) to collect and submit ready-to-eat (RTE) meat and poultry product samples to FSIS laboratories and, when appropriate, to document noncompliance in response to positive test results. Instructions concerning Lm verification activities other than sampling and responses to positive results are contained in FSIS Directive 10,240.4, Listeria Rule Verification Activities.
- FSIS is reissuing this directive to reflect changes to product sampling for Lm and Salmonella under a new combined RTEPROD project and updates to the sampling selection criteria for the new project to streamline communication. The directive includes RTEPROD scheduling information in the Attachment. Both post-lethality exposed and not post-lethality exposed products are subject to RTEPROD sampling under the new combined program. Establishments will continue to be sampled at least two times per year and no more than 1 time per month (maximum of 12 samples per year per establishment).
- The directive clarifies the instructions related to submission of products containing meat and non-meat ingredients. FSIS is clarifying this language in response to an increase in discards of these products due to insufficient weight.
- The directive revises the instructions for products that receive a lethality treatment at another federally inspected establishment. These revisions are consistent with the instructions in the directives covering Shiga toxin-producing Escherichia coli (STEC) in raw beef product sampling and Salmonella in raw poultry sampling.
- The directive revises the instructions to clarify when to collect samples that have undergone High Pressure Processing (HPP). These revisions are consistent with the instructions in the directive covering Salmonella in raw poultry sampling.
KEY POINTS:- Collecting and submitting FSIS verification samples under the revised RTEPROD (sample project code for RTE product) sampling algorithm
- Collecting one-pound samples of RTE product for sample submission to the FSIS laboratories is required
- Rotating through all the products produced by the establishment is important when collecting samples (i.e., post-lethality exposed and not post-lethality exposed products, products produced under different alternatives, and also different product types)
- Taking enforcement actions in response to a positive sample result and verifying product disposition
- CANCELLATION
FSIS Directive 10,240.3, FSIS Ready-To-Eat Sampling Programs, 3/25/22 - BACKGROUND
- Under the Federal Meat Inspection Act (FMIA) and the Poultry Products Inspection Act (PPIA), FSIS considers any RTE product to be adulterated if it contains a pathogen of public health concern (depending on the type and level) or its toxin that can cause illness in humans. There are some pathogens where any level would make the RTE product adulterated (such as Lm and Salmonella) because presence of the pathogen could be injurious to health (21 U.S.C. 601(m)(1) and 453(g)(1)). If any level of Lm or Salmonella is detected in an RTE product or on a food contact surface (FCS) that RTE product has passed over, the product is adulterated.
- FSIS collects samples for its RTE sampling program under the RTEPROD sampling project.
- FSIS analyzed the results from the RTEPROD_RISK and RAND programs and determined that it would streamline communication to IPP and establishments to combine the programs into a new RTEPROD project. Both post-lethality exposed products and not post-lethality exposed products are eligible for sampling under the RTEPROD sampling program. Not post-lethality exposed products are included in the sampling program even though they are not subject to the Listeria Rule requirements in 9 CFR 430 because FSIS identifies Lm positives in these products and several recent listeriosis outbreaks have been associated with products that were incorrectly classified as not post-lethality exposed. The criteria for assigning RTEPROD sampling tasks can be found in the Attachment.
CHAPTER II - FSIS RTE SAMPLING
- PRODUCTS SUBJECT TO RTEPROD SAMPLING
- All RTE meat and poultry products are subject to RTEPROD sampling except those listed in G. of this section.
- To determine product sampling eligibility, IPP are to consider if the establishment's hazard analysis intended use statement, and flow chart, and Hazard Analysis and Critical Control Point (HACCP) plan, are consistent with production of an RTE product. According to FSIS Directive 5,300.1, Managing the Establishment Profile in the Public Health Information System (PHIS), FSIS considers products in the Fully Cooked – Not Shelf Stable HACCP category to be RTE. HACCP categories that may contain either RTE or not ready-to-eat (NRTE) products include Not Heat-Treated – Shelf Stable, Heat Treated – Shelf Stable, and Product with Secondary Inhibitors – Not Shelf Stable.
- FSIS considers a product to be RTE and subject to sampling if it meets one or more of the following criteria:
- The product meets the definition of an RTE product in the Listeria Rule (9 CFR 430.1). The Listeria Rule defines an RTE product as a meat or poultry product that is edible without additional preparation to achieve food safety. This includes products that have been processed to meet the requirements of 9 CFR 318.17, 9 CFR 318.23, or 9 CFR 381.150 or undergone other processing to render them RTE.
- IPP are to be aware that not all RTE products are required to meet a standard of identity. There is a standard of identity requiring that certain products be fully cooked according to 9 CFR 319 and 9 CFR 381 (e.g., hot dogs or barbeque). For other RTE product, the establishment identifies the intended use of the product as RTE based on consumer expectation and the product name (e.g., pâtés or deli meat).
NOTE: IPP are to be aware that the establishment may consider certain products (e.g., hams) as either RTE or NRTE if there is no standard of identity defining the product as RTE or the intended use is not typically RTE even if the product receives a full lethality treatment (e.g., meat casserole). Products that receive a full lethality treatment but are classified by the establishment under a NRTE HACCP plan, are not eligible for FSIS sampling under RTEPROD (e.g., hams, tamales). - The product is not labeled with safe handling instructions (SHI), as required for NRTE products by 9 CFR 317.2(l) and 9 CFR 381.125(b). According to 9 CFR 430.1, RTE products are not required to bear SHI or other labeling that directs that the product be cooked or otherwise treated for safety (although RTE products may bear heating instructions). FSIS considers products labeled with SHI and cooking instructions to be NRTE and not subject to sampling under the RTE sampling projects.
- Both post-lethality exposed products and not post-lethality exposed RTE products are eligible for sampling under the RTEPROD sampling program. Although the Listeria Rule (9 CFR 430) does not apply to not post-lethality exposed products, these products are subject to FSIS sampling under RTEPROD.
- Therefore, IPP are not to cancel RTEPROD samples just because an establishment only produces not post-lethality exposed products.
- The following are products eligible for RTEPROD sampling:
- Post-lethality exposed meat and poultry product;
- Not post-lethality exposed meat and poultry product. Examples of not post-lethality exposed products include those that are:
- Cooked in a moisture impermeable bag and remains in the cooking bag until it enters commerce (e.g., cook-in-bag product; sous-vide is a type of cook-in-bag). IPP are to be aware that:
- If the establishment punctures the impermeable bag (e.g., with a thermometer) and repackages and reprocesses the product in another impermeable bag without punctures or holes before distributing it, the product can continue to be categorized as not post-lethality exposed.
- If the establishment punctures the impermeable bag (e.g., with a thermometer or because it perforates or punches small holes in the bag for product quality) and the establishment does not repackage and reprocess the product in another impermeable bag without punctures or holes, then the product is considered post-lethality exposed.
- Hot-filled (e.g., soup) at a temperature sufficient to achieve full lethality of the product (e.g., using one of the time/temperature combinations in the FSIS Cooking Guideline for Meat and Poultry Products (Revised Appendix A).
- Cooked in a moisture impermeable bag and remains in the cooking bag until it enters commerce (e.g., cook-in-bag product; sous-vide is a type of cook-in-bag). IPP are to be aware that:
- Post-lethality and not post-lethality exposed meat and poultry product labeled "For Further Processing," in which the product does not receive a lethality treatment at another federally inspected establishment;
- Popped pork skins, pork rinds, dried soup bases, concentrated (high salt content) soup mixes, and pickled pig's feet;
- Products that are hot shipped, such as pasties, hot meat pies, or convenience meals that are cooked and shipped hot without cooling;
- Products that will later be processed at an off-site establishment that apply a treatment, such as HPP, which is used to achieve food quality characteristics or to extend product shelf life and not as support for decisions made in their HACCP system, per Directive 5000.15, Verification Activities for High Pressure Processing, Irradiation and Microwave Tempering;
- Products that will later be processed at an off-site establishment that applies a treatment, such as HPP, as an intervention , which is used to reduce or eliminate an adulterant, such as Lm or Salmonella, and support a decision in the HACCP system, per Directive 5000.15; and
NOTE: While products treated with HPP are eligible for sampling regardless of why the HPP is applied (for quality or food safety) or whether the product is returned to the producing establishment or not, these factors do impact when and where FSIS collects the sample. For further instructions on when to collect RTEPROD samples of product treated with HPP see Section II.D. - Siluriformes fish as instructed in Directive 14,010.1, Speciation, Residue, and Salmonella Testing of Fish of the Order Siluriformes from Domestic Establishments.
- The following are products are ineligible for RTEPROD sampling. For RTEPROD sample requests, IPP are not to collect samples of:
- Pass-through product, which is fully packaged finished product that the establishment has received and kept in its package without further post-lethality exposure, processing, or repackaging. For example, pass-through products, such as pre-packaged deli meat that the establishment leaves in the package and combines with separately packaged cheese and crackers that are not comingled or touching, are not to be sampled.
- Oils, shortening, lard, margarine, oleomargarine, or mixtures of rendered animal fats because there is no validated method for testing these products for Lm.
- IPP are to ensure lards and oils are appropriately entered into the PHIS profile so that sampling tasks are not assigned in establishments that only produce lards/oils.
- IPP are to enter the products under the HACCP Category of Heat Treated-Shelf Stable, the Finished Product Category of RTE dried meat, and the Product Group as Lard/oils. For information on how to update the PHIS profile, see FSIS Directive 5,300.1.
- Product labeled "For Further Processing," in which the product will receive a lethality treatment at another federally inspected establishment.
- IPP are not to sample product that will receive a lethality treatment at another federally inspected establishment.
- IPP are to verify that the establishment's hazard analysis and flow chart show that the product is intended for receiving a lethality treatment at another federally inspected establishment. If not, IPP are to collect the sample.
- THE SAMPLED LOT
- The sampled lot is product that is represented by the sample FSIS collects and analyzes for Lm and Salmonella. The establishment is responsible for defining the sampled lot.
- FSIS generally considers the sampled lot to be the product produced from "clean-up to clean-up," unless the establishment has a different supportable definition of the lot (e.g., products that are produced on different lines and that are microbiologically distinct from one another).
- An official establishment may reduce its lot size on a day when FSIS collects a routine RTE sample to facilitate holding the product if the change does not interfere with FSIS' ability to collect a representative sample.
NOTE: For example, an establishment that normally produces product over an 8-hour shift, followed by a complete clean-up, may reduce its lot size when FSIS collects a sample. The establishment may then produce product over a 4-hour period, followed by a complete clean-up. - There are other options that establishments may use to reduce lot size, if FSIS can still collect a representative sample. Instructions to verify an establishment's written sampling program design and execution can be found in FSIS Directive 10,240.4, Listeria Rule Verification Activities, Chapter III.
- IPP are to be aware that establishments may reduce the lot size even when using source materials that are post-lethality exposed and do not undergo further lethality treatment. The establishment is not required to hold other lots using the same source materials because the sampled lot is those products produced from clean-up to clean-up.
- For example, if an establishment reduces the lot (as outlined in C.1. of this section) in the production of prepared chicken salad using RTE post-lethality exposed chicken from another supplier, the establishment may reduce its lot size to a 4-hour period of chicken salad production, followed by a complete clean-up. The establishment can make another lot of chicken salad using the same source materials and not hold that lot. In the event of a positive, the establishment will need to provide a scientific basis to justify why the other lots should not be implicated.
- IPP are to be aware of the difference between the sampled lot and the implicated lot in the event of a positive.
- The sampled lot is product that is represented by the sample FSIS collects and analyzes for Lm and Salmonella. The establishment is responsible for defining the sampled lot.
- The implicated lot (or lots) is the product that may be connected to a sampled lot that tested positive through common source material or other root cause findings as described below. The implicated lots are determined by root cause findings and may be defined through investigations by FSIS, other public health agencies, the establishment, or foodborne illness findings.
- The establishment is required to retain HACCP records for the time period specified in 9 CFR 417.5 documenting the product code, product name or identity, or slaughter lot. The product code is used by the establishment to identify a particular lot of product and is needed to identify the implicated lots should the establishment need to recall additional product made using positive source materials.
- IPP are to consider the impact that decreasing the lot size may have on sample collection. FSIS recommends samples be collected at least 3 hours into operations, if possible, to allow Lm to work its way out of the equipment. As a result, if the establishment produces a very small lot on the day FSIS collects a sample when it typically produces a larger lot, then FSIS may not be able to collect a representative sample. In this case, IPP are not to collect a sample and are to reschedule the sample for another day. If the establishment typically produces RTE product for less than 3 hours, then the samples can be collected less than 3 hours into operations.
- IPP are to ensure that establishments do not reduce the lot size to a single piece of one-pound product (e.g., a single deli chub) or other unrepresentative lot size. A representative sample does not mean a lot that is comprised of a single one-pound piece of product.
- As stated in B. above, FSIS generally considers the sampled lot to be the product produced based on the establishment's supported lot definition or from "clean-up to clean-up." However, in the event of a positive result or harborage findings, additional product may be included in the implicated lot.
- The implicated lot may include other products using the same RTE source materials:
- If an establishment uses RTE source materials received from another establishment, and there is reason to conclude that those products are the source materials for a Lm positive, additional product may be included in the lot, outside the establishment's clean-up to clean-up lotting procedures (e.g., if there are positive test results for an individual source material).
- For example, if the establishment uses a RTE chicken source material to make different lots or types of chicken salad, and FSIS sampling finds a Lm positive in the chicken and it matches a Lm positive in the chicken salad by Whole Genome Sequencing (WGS), then all the different lots of chicken salad that used the same RTE chicken source material would be part of the implicated lot.
- Ingredients (e.g., pepper or other spices) added to post-lethality exposed RTE products can affect the lot definition. The establishment is required to evaluate the possible hazards from all ingredients it uses, as per 9 CFR 417.2(a)(1).
- The implicated lot may include other products using the same processing steps:
- If the root cause of the positive is due to under-cooking or under-processing, then other products using the same processing method can be implicated. Since Salmonella can contaminate RTE products because of under-processing, the adequacy of the lethality step may be in question.
- For example, if one lot of RTE product tests positive by FSIS and the root cause identified under-cooking, and a subsequent lot of product received the same lethality treatment, a scientific basis is necessary to justify why the later lot should not be included in the implicated lot.
- The establishment's brine, used to chill product, is reused across lots and can cross-contaminate the lots and prevent them from being microbiologically distinct.
- Harborage findings:
- Harborage or reintroduction of Lm occurs when Lm persists in the processing environment over time. Harborage may be identified based on FSIS test results when closely related Lm isolates (as determined by the Office of Public Health Science (OPHS) using WGS) are found in product, food contact, or environmental samples collected over multiple days, weeks, months, or years.
- Evidence of harborage may indicate insufficient sanitary measures to prevent contamination of the production environment and the products with Lm and may result in additional product associated with the lot, outside the establishment's clean-up to clean-up lotting procedures.
- Cross-contamination findings: Cross-contamination occurs when Lm moves among food, FCS, or non-food contact environmental surfaces in the establishment. Cross-contamination is identified based on FSIS test results when closely related Lm isolates (as determined by OPHS using WGS) are found in product, food contact, and/or environmental (non-food contact) samples collected during the same sampling event. If Lm is isolated from a post-lethality exposed product sample and from an FCS sample, the FCS is more likely to be the source, unless under-processing of RTE product is suspected.
- The implicated lot may include other products using the same RTE source materials:
- If IPP have questions about whether an establishment is altering routine production, sanitation, or food safety practices, they are to discuss the issue with their supervisor, and if additional help is needed, can submit questions through askFSIS following the instructions in Chapter VII, Questions.
- IPP are to be aware of the following factors or conditions that may determine a sampled lot:
- Frequency of cleaning and sanitizing: The establishment may perform a complete cleaning and sanitizing (following the procedures in its Sanitation Standard Operating Procedure (Sanitation SOP)) to differentiate lots.
- Separation between processing lines:
- Products produced in the same room can be considered part of the same lot or different processing lots, depending on how the lots are separated by time and space.
- Products produced on different processing lines can be considered different lots if the lines are microbiologically and physically independent (e.g., equipment, personnel, utensils, and RTE source materials are not shared among the lines).
- Products produced on the same line can be considered different processing lots if their production is separated by complete cleaning and sanitizing, and if they differ according to the other factors described above.
- Products stored in a common cooler would not necessarily be considered part of the same lot. IPP are to be aware that the establishment's Sanitation SOP should address possible cross-contamination if exposed products from different lots are stored in the same cooler.
CHAPTER III – COLLECTING AND SUBMITTING FSIS VERIFICATION SAMPLES
- SCHEDULING THE SAMPLE
- IPP are to discuss sample scheduling with the establishment at the weekly meeting and document the discussion in a Memorandum of Interview (MOI), as described in FSIS Directive 5,000.1, Verifying An Establishment's Food Safety System. As part of this discussion, IPP are to determine:
- The types of RTE products produced by the establishment; and
- How much notice to give the establishment when collecting a sample. IPP are to familiarize themselves with the establishment's production practices so that they can provide adequate time to allow the establishment to hold all product represented by the sample, (i.e., the sampled lot) but not alter its production practices.
- When IPP receive an RTEPROD request in the PHIS, they are to schedule sample collection within the sampling window timeframes given.
- IPP are to add the sampling task to the task calendar and set up a collection date and parcel pickup date, in accordance with FSIS Directive 13,000.2, Performing Sampling Tasks in Official Establishments Using the Public Health Information System. Any rescheduled or canceled sampling tasks are to be recorded in PHIS.
- IPP are not to wait until the end of the sampling window to schedule the sample. Scheduling the sample at the beginning of the sampling window will allow more time to ensure that the sample is available, and that capacity is available at the labs during the sampling window.
- To schedule the sample, IPP are to randomly select a day, shift, and time within the sample window timeframe.
- IPP are to schedule samples from all shifts in which the establishment produces RTE products. There should be an equal chance that sampling will occur during any shift where eligible product is produced.
- If IPP try to schedule a sampling task, but PHIS says there is "no lab capacity available," they are to consult IPP Help, Requesting Lab Capacity.
- Before collecting a sample, to provide establishments enough time to hold the entire sampled lot, but not enough time to alter their production practices, IPP are to:
- Generally, provide one day's notice if such advanced notice is sufficient for the establishment to hold the sampled lot, but not to change practices. IPP may provide two days' notice, if necessary.
- Consider the establishment's request for more than two days' notice, in the rare case that more notice is needed based on the establishment's product and process flow. If the establishment can support that more notice is necessary because of the innate characteristics of the process (e.g., less-than-daily sanitation, use of brine, or processes that span more than two days), IPP may provide more than two days' notice. If IPP have questions about an establishment's basis for requesting more notice, they are to discuss them with their supervisor, and if additional help is needed, are to submit them through askFSIS following the instructions in Chapter VII, Questions.
- Inform the establishment that if routine practices are changed without justification for doing so, FSIS may provide less than one day's notice, if less time is sufficient to hold the sampled lot, but not change routine practices.
- Inform the establishment that it is responsible for supporting the basis for defining the product represented by the sample (i.e., the sampled lot); and
- Inform the establishment that it is required to hold or control the sampled lot when FSIS collects RTE products until negative results become available.
- When notifying the establishment that FSIS will collect a sample, IPP are to:
- Confirm the establishment will be producing applicable product on the day sampling is scheduled;
- Confirm the establishment is planning to implement its documented routine production, Sanitation SOP, and food safety practices on the day the sample is scheduled; and
- Inform the establishment that, if it intends to modify its documented routine production, sanitation, or food safety practices before the sampling, the establishment should inform IPP as soon as possible, so that sampling can be rescheduled.
- If the establishment continues to change routine practices and cannot support the changes, noncompliance is to be documented as specified in Chapter IV, Documenting Noncompliance. IPP are to also work through supervisory channels to request a Public Health Risk Evaluation (PHRE), as appropriate (FSIS Directive 5,100.4, Enforcement, Investigations, and Analysis Officer (EIAO) Public Health Risk Evaluation (PHRE) Methodology).
- Justifiable reasons for changing practices may include limiting the lot size to facilitate holding the product, changes in customer orders, or documented changes to Sanitation SOPs or HACCP plans.
- At the next weekly meeting, IPP are to discuss with the establishment the changes to routine production, sanitation, or food safety practices. IPP are to inform the establishment that if it continues to change its practices, FSIS may collect more samples or give less than one day's notice.
- In PHIS, after collecting the sample, IPP are to:
- Verify that the establishment is holding or controlling the product represented by the sampled lot and record the information in PHIS under the Sample Collection Data tab as:
- "Yes," if product is held on-site or off-site under company control; or
- "No," if the sampled lot was not held or controlled by the establishment because the product was denatured on-site or because the establishment did not wait to complete pre-shipment review following availability of all relevant test results, as set out in 9 CFR 417.5(c).
- Immediately contact the District Office (DO) through supervisory channels if the establishment does not hold or maintain control of the sampled lot and the sampled lot was not denatured on-site.
- Verify that the establishment is holding or controlling the product represented by the sampled lot and record the information in PHIS under the Sample Collection Data tab as:
- IPP are to discuss sample scheduling with the establishment at the weekly meeting and document the discussion in a Memorandum of Interview (MOI), as described in FSIS Directive 5,000.1, Verifying An Establishment's Food Safety System. As part of this discussion, IPP are to determine:
- COLLECTING THE SAMPLE
- IPP are to randomly (without priority order) select a product produced at the time the sample is scheduled, regardless of whether the product is post-lethality exposed or not. IPP are to make efforts to cycle through all the products produced by the establishment (i.e., post-lethality exposed and not post-lethality exposed products, products produced under different alternatives, and also different product types). If the product tests positive, IPP are to consider the establishment's hazard analysis and supporting documentation prior to issuing a noncompliance record (NR) as described in Chapter IV, Documenting Noncompliance.
- IPP are to collect one pound of RTE product. The labs require at least 1 pound of product to analyze the sample for Lm and Salmonella. IPP are to collect either one pound of meat or poultry only or one pound of a complete product, including meat or poultry and non-meat or poultry components when the ingredients are commingled. Failure to collect the minimum amount will result in a sample discard.
- When the product contains meat or poultry and non-meat or poultry ingredients, IPP are to review IPP Help, multi-component RTE Product Sampling, which contains examples and photos of how to determine how much product to collect; IPP Help also includes photos of commingled (in contact) and non-commingled (not in contact) ingredients in final packaging (i.e., packaging that is normally shipped by the establishment into commerce). IPP are to ensure that:
- If the meat or poultry and non-meat or poultry ingredients are commingled (in contact) in the final package (e.g., a salad with meat or poultry mixed in, bread product stuffed with meat), IPP are to collect a one-pound sample of the complete final product (including the meat or poultry and non-meat or poultry component).
- If the meat and non-meat ingredients are not commingled (not in contact) in the final package (e.g., an entree with separate compartments for meat or poultry and vegetables), then IPP are to collect enough final packages to reach the one-pound sample weight for the meat or poultry component, or the establishment may slack-fill the meat or poultry component only into the final package. Other components that are non-meat or poultry do not count towards the sample weight in non-commingled products. Generally, multiple entrees are necessary to ensure there is sufficient meat or poultry available for laboratory testing.
- IPP are to collect the sample after the establishment has applied all interventions except any microbiological testing. If the establishment intends to test the product for Lm or Salmonella, IPP are not to wait for the establishment to receive the test results before collecting a sample.
- If the establishment treats the product with an intervention (e.g., HPP), either at the establishment or at another establishment (off-site), IPP are to review the documentation that the establishment keeps as part of its HACCP program to verify whether the purpose of the treatment is to reduce or eliminate an adulterant in a RTE product, such as Lm or Salmonella, or to extend shelf-life. For more information on the different uses of HPP, IPP are to refer to Directive 5000.15.
- As indicated in Section I.F., products that are treated with HPP, whether for a lethality treatment or to extend shelf-life are eligible for RTEPROD sampling. IPP are to consider the following when collecting samples of products treated with HPP:
- If off-site interventions, such as HPP, are applied to reduce or eliminate an adulterant in a RTE product, such as Lm or Salmonella, and support a decision in the HACCP system, IPP are to sample such products after they return to the producing establishment after the off-site intervention has been applied.
- If off-site interventions, such as HPP, are applied to reduce or eliminate an adulterant, such as Lm or Salmonella, and the product is not returned IPP are to contact their Office of Field Operations (OFO) Supervisor for instruction. The Frontline Supervisor is to work with the District Office to ensure samples are collected. IPP are not to collect the sample at the HPP establishment.
- If the establishment treats the product with an off-site treatment such as HPP to achieve food quality characteristics or to extend product shelf life and not as support for decisions made in their HACCP system, IPP are to collect a sample in the final packaging at the producing establishment before the product is shipped off-site for the treatment.
- IPP are to collect the product at least three hours after the start of production, whenever possible, to allow Lm to work its way out of the equipment. If the establishment's production lot is typically less than three hours, IPP may collect the samples during the production shift. IPP may collect samples on the first shift or second shift (or other shifts, as applicable). IPP are to vary the shifts in which they collect samples, if possible.
- IPP are to collect a one-pound sample of product in the final packaging (i.e., packaging that is normally shipped by the establishment into commerce). Collecting products in the final package will help ensure that the product does not become contaminated with Lm from the environment during the sample collection process. A one-pound sample is needed for all products, including jerky, because FSIS tests products for multiple analytes.
- If the establishment produces reworked product, IPP are to sample the product as part of the production lot, as long as IPP provide the establishment with adequate notice to hold the sample.
- IPP are to be aware that FSIS collects samples in the final package after all interventions are complete, even if the establishment has recooked, reprocessed, or repackaged the product.
- IPP are to submit the samples to the laboratory for microbiological analysis in the final package. The laboratory does not supply sterile bags or gloves for sampling because IPP are not to have direct contact with the exposed, unpackaged RTE product so that there is no opportunity for IPP to contaminate the sample. This is because Listeria may be present in the environment and could be transferred to the product if an exposed RTE product is collected. The laboratories disinfect intact retail packages at the incision sites prior to incision for sampling. However, for RTE sausages in casing, the shell/casing is an integral part of the sample and should be free of pathogens and toxins. Therefore, as indicated in the Microbiology Laboratory Guideline (MLG) 8.14, the casing is not disinfected since some casings are permeable and the disinfectant may be introduced into the core of the product. In addition, consumers often slice through an inedible casing and then remove it thus any contamination on the surface of the casing could be transferred to the edible core of the product.
NOTE: Final packaging may include butcher paper, wax paper, plastic wrap, or any packaging that is not sealed. - If the final package or product container is too large, heavy, or costly to ship to the laboratory or the establishment only ships product in bulk, IPP can contact the laboratory through PHIS to request a larger shipping container or ask the establishment to slack-fill or short-weight a product for a one-pound sample and send it in the usual establishment packaging, such as the container liner. IPP are not to cut the product to fit it inside the shipping container. The following are additional instructions regarding slack-filling or short-weighting:
- If possible, IPP are to ensure the establishment slack-fills or short-weights a one-pound sample in the usual establishment packaging and seal it (e.g., vacuum seal).
- If the product is shipped in bulk using a liner bag inside a box, IPP are to ensure the establishment slack-fills or short-weights a one-pound sample into the container liner. IPP are to tie off the liner bag (e.g., by knotting the bag or using a rubber band) so smaller particles (e.g., shredded meat pieces) or liquid does not spill into the shipping container. IPP are to place the slack-filled package in a secondary bag. The laboratory will discard the sample if it contains spilled or leaking products.
- If the product is shipped in bulk and there is no liner bag (e.g., a wax lined box), IPP are to ensure the establishment slack-fills or short-weights a one-pound sample using its bulk packaging (e.g., the wax lined box with no liner bag) or the establishment may use food-grade packaging or sterile packaging such as Whirl-Pak bags. Laboratory-supplied bags (e.g., zip top bags) provided for FSIS RTE sampling are for secondary containment to protect the shipping container from possible sample leakage and are not sterile. The laboratory-supplied bag protects the box in case the primary container leaks.
- IPP are not to slack-fill the sample and are not to supply the establishment with a laboratory-supplied bag as the primary wrap or container for the sample. The establishment is responsible for slack-filling the product in packaging that they supply.
- When IPP document the sampling task in PHIS, under the Additional Info tab, they are to click "yes" to the question "Is this sample short-weighted/slack-filled?" to ensure that the sample is not discarded by the laboratory. Per this directive, IPP are to ensure the sample is short-weighted or slack-filled by the establishment employees or equipment in establishment-supplied packaging.
- If submitting samples of products that contain lactic acid starter cultures, such as dry and semi-dry fermented sausages, IPP are to answer "yes" to the question "Does this sample contain a lactic acid starter culture?" under the Additional Info tab in PHIS. The laboratories use this information to determine the correct method of sample preparation, which differs for products containing lactic acid starter culture as described in the Microbiology Laboratory Guideline (MLG) 4.14.
- SUBMITTING THE SAMPLE
- IPP are to safeguard the integrity of samples during submission according to FSIS Directive 7,355.1, Use of Sample Seals for Laboratory Samples and Other Applications.
- IPP are to ship samples to the designated laboratory as soon as collected and during the next available FedEx pickup. IPP are to ship samples refrigerated or frozen, depending on establishment practices. IPP are to use sufficient frozen gel packs to keep samples cold during transit. IPP are to ship samples Monday through Friday. IPP are not to ship samples on Saturdays or on the day before a Federal holiday, or as directed by an Agency user notice via e-mail notification.
- According to FSIS Directive 13,000.2, IPP are to submit information through PHIS to transfer electronic records to the lab. To submit samples to the lab, IPP are to apply the bar code label from the sample seal set to the designated location at the top of the lab form and sign and date the form before placing it in the shipping container. Additional information on the use of sample seals can be found in FSIS Directive 7,355.1.
- IPP are to respond in a timely manner to any requests from the FSIS laboratories regarding sample or form information (e.g., if the sample is missing a form that IPP need to submit) to avoid the sample being discarded.
- IPP are to use Table 1 on the following page to reference RTE sampling instructions.
Table 1: Summary of RTE Sampling Instructions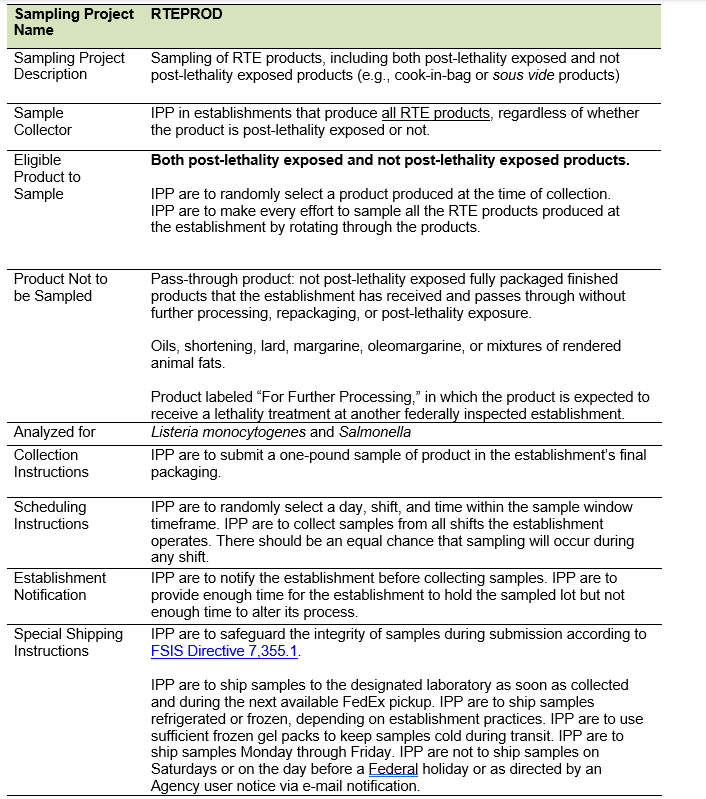
CHAPTER IV – DOCUMENTING NONCOMPLIANCE
- ESTABLISHMENT TEMPORARILY CHANGES PRACTICES
- IPP are to issue an NR under the following circumstances:
- If IPP find that the establishment has made changes in its food safety systems on the day the sample is collected (e.g., temporarily changing its supplier of RTE product or purchasing new source material for the sampled lot) and does not have documents supporting the appropriateness of the change, IPP are to issue an NR. The NR would be recommended because the establishment did not consider the changes in its hazard analysis in accordance with 9 CFR 417.2(a)(1) or did not support the changes to its hazard analysis as in 9 CFR 417.5(a)(1).
- Likewise, if IPP find that the establishment has made changes in its sanitation practices (e.g., temporarily increasing the use of sanitizer only on the day the sampling is scheduled) and did not revise its Sanitation SOP to reflect these changes, IPP are to issue an NR under 9 CFR 416.14.
- IPP are to issue an NR under the following circumstances:
- SAMPLING RESULTS FROM RTEPROD
- Sampling results will be reported to IPP in PHIS. IPP are to review the testing results and inform the establishment of the results, according to FSIS Directive 5,000.1.
- Whenever IPP are notified that a sample has been discarded and will not be analyzed by the FSIS laboratory, and product is being held on-site or controlled off-site, IPP are to notify the establishment immediately so the product can be released.
- FSIS will withhold its determination as to whether meat and poultry products are not adulterated, and thus eligible to enter commerce, until all FSIS test results that bear on the determination have been received.
- If an RTE product sample collected by IPP tests positive for Lm or Salmonella, product from the sampled lot is considered adulterated. IPP are to follow the instructions in FSIS Directive 5,000.1 to take regulatory action in response to positive sampling results. For information on product disposition options see Chapter V, Verifying Product Disposition.
- If FSIS finds the product to be positive and the establishment tested the product under its documented sampling programs, IPP are to check the establishment's Lm or Salmonella testing results to determine whether the establishment also found the sampled product to be positive for Lm or Salmonella.
- IPP are to determine whether the establishment held the product or otherwise maintained control of the product (e.g., the establishment moved the product off-site but did not complete pre-shipment review or transfer ownership of the product to another entity) pending FSIS test results. If IPP find that the establishment did not hold or maintain control of the product, they are to issue an NR because the establishment shipped product before FSIS found that the product was not adulterated, and because the establishment did not complete pre-shipment review following availability of all relevant test results, as set out in 9 CFR 417.5(c). IPP are to immediately contact the DO through the supervisory chain of command. If the results are confirmed positive for Lm or Salmonella, the DO is to take appropriate regulatory action and contact the Recall Management and Technical Analysis Division (RMTAD) and Office of Investigation, Enforcement and Audit, Compliance and Investigation Division (CID), Regional Director (RD). As appropriate, FSIS will request a recall or detain the product. The CID RD, in consultation with Headquarters, will consider whether additional enforcement actions or sanctions are necessary.
- Generally, if FSIS finds the product positive for Lm or Salmonella, IPP are to issue an NR (cite 9 CFR 301.2 for meat, 9 CFR 381.1 for poultry, and 9 CFR 417.4(a)) because the establishment's HACCP system did not identify the adulterated product being produced. However, if the establishment collected its own sample from the same sampled lot of product also found their sample to be positive for Lm or Salmonella and held the product, IPP are not to issue an NR. They are to verify that the establishment performs the appropriate corrective actions, using a directed HACCP Verification Task.
- IPP are to be aware that WGS is performed on all Lm and Salmonella isolates and Lm results are shared with DO personnel as described in FSIS Directive 10,240.6, Use of Whole Genome Sequencing Results for FSIS RTE Sampling Programs.
- VERIFYING CORRECTIVE ACTIONS IN RESPONSE TO AN FSIS POSITIVE RESULT
- If FSIS finds a product positive for Lm or Salmonella under the RTEPROD program, IPP are to verify that the establishment takes the appropriate corrective actions by performing a directed HACCP Verification Task.
- When performing a directed HACCP Verification Task in response to a Lm positive result, IPP are to review the same information they review during a routine HACCP Verification Task.
- IPP are also to verify that the establishment implemented corrective actions according to 9 CFR 417.3(a) or (b) if the measures for addressing Lm are included in the HACCP plan or prerequisite program, or 9 CFR 416.15 if the measures are incorporated in the Sanitation SOP.
- FSIS will perform a PHRE for Lm, as described in FSIS Directive 10,300.1, Intensified Verification Testing (IVT) Protocol for Sampling of Product, Food Contact Surfaces, and Environmental Surfaces for Listeria monocytogenes (Lm).
- If the establishment considers Listeria not reasonably likely to occur because the establishment has a prerequisite program, IPP may also perform a directed HAV task as described in FSIS Directive 5,000.6, Performance of the Hazard Analysis Verification (HAV) Task to verify the establishment can continue to support its decisions in its hazard analysis.
- When performing a directed HACCP Verification Task in response to a Salmonella positive result, IPP are to verify that the establishment took the appropriate corrective actions according to 9 CFR 417.3(a) or (b), or 9 CFR 416.15. As stated previously, FSIS considers RTE products to be adulterated if products or FCS test positive for either Lm or Salmonella. Therefore, establishments are required to take corrective actions in response to positive results and to reassess their HACCP plan if they haven't addressed these hazards. FSIS will perform a PHRE in response to Lm or Salmonella positives, as described in FSIS Directive 5,100.4.
NOTE: IPP are to be aware that establishments should take action in response to multiple Listeria positives that show relatedness through whole genome sequencing results. A trend of related positives may be an indicator of Listeria harborage. - If FSIS develops a verification plan (under FSIS Directive 5,100.3, Administrative Enforcement Action Decision-Making and Methodology) in response to an establishment's corrective actions and preventive measures, and enforcement is deferred following the issuance of a Notice of Intended Enforcement (NOIE) or a suspension is held in abeyance, IPP are to verify that the establishment implements its corrective actions, and that the corrective actions are effective.
- IPP are to verify that the establishment took the following actions:
- If Lm control is addressed as a Critical Control Point (CCP) in the HACCP plan (e.g., because they are using a post-lethality treatment or PLT), the establishment must meet the requirements of 9 CFR 417.3(a), which requires that corrective action be taken but does not require reassessment of the HACCP plan.
- If Lm is addressed in the Sanitation SOP, then the establishment must implement corrective actions in accordance with 9 CFR 417.3(b), which includes reassessment of the HACCP plan. In addition, it is to implement the corrective action requirements for the Sanitation SOP in 9 CFR 416.15, which includes appropriate reevaluation or modification of the Sanitation SOP.
- If Lm is addressed in a prerequisite program (e.g., Listeria control program) that is used to support the decision that Lm is not a hazard reasonably likely to occur in the product, then the establishment must implement the corrective actions in 9 CFR 417.3(b) and comply with 9 CFR 417.4(a)(3). As part of this, the establishment must perform a HACCP reassessment to determine whether the newly identified deviation or other unforeseen hazard should be incorporated into the HACCP plan.
- The establishment is required under 9 CFR 417.4 (a)(3) to document the reassessment and the reasons for any changes that it made to its HACCP plan as a result of the reassessment, or, if it did not make any changes, to document the reasons why it did not.
- If an establishment reclassifies an RTE product as a NRTE product in its HACCP plan in response to a positive result, IPP are to verify that:
- The product is not defined by a standard of identity as fully cooked (e.g., hot dogs) or the intended use is not typically RTE (e.g., pâtés or deli meats). If an establishment identifies the intended use as NRTE for products such as pâtés, deli meats, pepperoni, salami, bresaola, biltong, and droëwors where the intended use is typically RTE, the establishment must have on-file documentation supporting their decisions (9 CFR 417.5(a)(1)). This support must address how the establishment can ensure the consumer will properly cook the product (9 CFR 417.5(a)(1)), particularly if there is evidence such as marketing materials or recipes commonly indicating the product is RTE.
- The establishment labels the product as one that is NRTE and requires validated cooking instructions for safety so that the product label is accurate and not misleading, in compliance with 9 CFR 317.8 or 381.129. For example, use of the terms "Baked" or "Broiled" on the label of a NRTE product (e.g., baked chicken on the label) would be false and misleading because they indicate that the product is cooked and, therefore, suggest to the consumer that the product is RTE.
- The establishment has chosen a HACCP category consistent with that for a NRTE product. As explained in FSIS Directive 5,300.1, Attachment 1: HACCP Processing Categories, FSIS regards products in the Fully Cooked – Not Shelf Stable processing category as RTE. Therefore, categorizing the product in a Fully Cooked – Not Shelf Stable HACCP processing category would not make it a NRTE product.
- The establishment clearly identifies the intended use of the product in the flow chart or hazard analysis according to 9 CFR 417.2(a)(2). For the description to be consistent with that for an NRTE product, the establishment must describe the customary preparation practices for the safe consumption of the product. The establishment should also state why these practices can be regarded as customary preparation.
- The establishment takes corrective actions (e.g., intensified cleaning and sanitizing) and maintains sanitation in its environment according to 9 CFR 416.4(b) so that insanitary conditions, leading to product contamination, do not exist.
Figure 2. Steps for Verifying an Establishment's Corrective Actions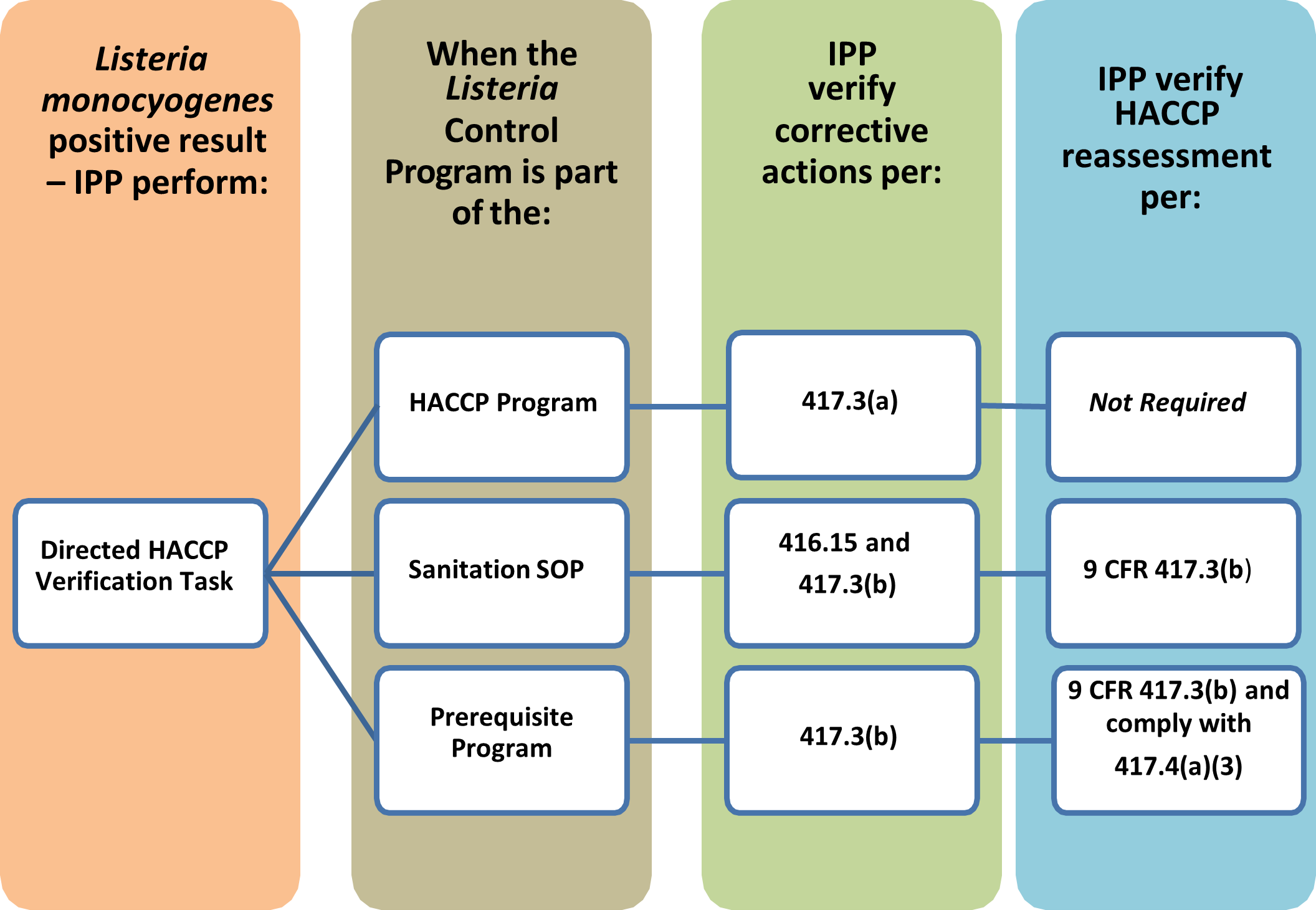
- If the establishment decides to produce not post-lethality exposed (e.g., cook-in-bag product) in response to a positive Lm result from FSIS testing under the RTEPROD program, IPP are to verify that the establishment:
- Revises its flow chart or hazard analysis according to 9 CFR 417.2(a)(2) to include the step for not post-lethality exposed RTE product (e.g., the step in which the product is cooked in the bag).
- For cook-in-bag products, ensures that the cooking bag is completely sealed (impermeable), so that moisture is contained within the bag or contaminants do not enter the bag. Cooking bags may be compromised during steps such as molding or shaping. The establishment should have a process to verify the package integrity, and if leakers are observed, to reprocess or recook the product.
NOTE: If the product is dried before cooking, it would not be appropriate to recook the product multiple times using the FSIS Cooking Guideline for Meat and Poultry Products (Revised Appendix A) as support for the process. For dried products that are cooked multiple times, the establishment would need to provide additional scientific support for the cooking process. - Uses a supportable process to recook the product to address potential cross-contamination from a thermometer stem if the establishment punctures the bag when taking the temperature of the product.
- The establishment takes corrective actions (e.g., intensified cleaning and sanitizing) and maintains sanitation in the processing environment, according to 9 CFR 416.4 to ensure that insanitary conditions do not exist, leading to product contamination.
CHAPTER V – VERIFYING PRODUCT DISPOSITION
- The establishment may reprocess or dispose of adulterated product. If the establishment reprocesses the product, IPP are to verify that it used a process that achieves adequate lethality of pathogens. FSIS considers a process that has been validated to achieve a 5-log reduction of Lm to be sufficient for reworking contaminated product.
- For cooked products, establishments may use the time-temperature tables in the FSIS Cooking Guideline for Meat and Poultry Products (Revised Appendix A) to recook the product.
- For dried products, it would not be sufficient to recook the product using the time-temperature tables in the FSIS Cooking Guideline for Meat and Poultry Products (Revised Appendix A), unless the establishment uses one of the following relative humidity options: Option 1, Option 3, Option 4, or Cook-in-bag, or Immersion cooking as supported by Scientific Gap #5.
- If the establishment chooses to dispose of the product, it may do so either on-site or off-site.
- If the product is disposed of on-site, IPP are to verify that the establishment maintains records showing that the positive product received the proper disposition.
- If the establishment transports positive product off-site for appropriate disposition, IPP are to verify that the establishment:
- Maintains records identifying the official establishment, renderer, or landfill operation that received positive product;
- Maintains control of product that was destined for a landfill operation or renderer while the product was in transit (e.g., through company seals);
- Maintains control of product that was destined for an official establishment while the product was in transit (e.g., through company seals) or ensured that such product moved under FSIS control;
- Maintains records showing that positive product received the proper disposition, including documentation showing proper disposal of the product from the official establishment, renderer, or landfill operation where disposition occurred;
- Completes pre-shipment review for the positive product only after it has received the records described above for that particular product; and
- If an establishment ships adulterated product to a renderer or landfill operation, IPP are to verify the establishment denatures the product before it leaves the establishment (9 CFR 314).
- If the establishment transports positive product to a pet food manufacturer, IPP are to verify the product is made inedible prior to shipment. IPP are to be aware that the product does not need to be denatured first, it could be placed in an inedible container and shipped under permit from the DO (9 CFR 314). IPP are also to be aware that the establishment is not required to maintain records showing that the positive pet food product received the proper disposition.
- If IPP find that there is noncompliance with the corrective action requirements for product disposal, they are to document the noncompliance in accordance with FSIS Directive 5,000.1.
- In situations where the establishment has not properly moved or disposed of the product, IPP are to notify their DO through supervisory channels.
CHAPTER VI – DATA ANALYSIS
FSIS will track Lm sampling data every year. The tracked data will include the number of samples scheduled, the number of samples collected, and the number of positives for each RTE project code. In addition, FSIS will track WGS results from RTE sampling programs and recalls from RTE meat and poultry products and will analyze these data to determine whether new policy is needed to address positive results.
CHAPTER VII – QUESTIONS
Refer questions regarding this directive to your supervisor or as needed to the Office of Policy and Program Development through askFSIS or by telephone at 1-800-233-3935. When submitting a question, complete the web form and select "Sampling" for the Inquiry Type.
NOTE: Refer to FSIS Directive 5,620.1, Using askFSIS, for additional information on submitting questions.
Attachment - Updates to Scheduling Criteria for the RTE Product Routine Sampling Program
>FSIS uses a statistical algorithm to assign RTEPROD sampling tasks at establishments that produce RTE products (both post-lethality and not post-lethality exposed). Typically, tasks are assigned on or around the 25th day of each month and are to be completed the following month. There is a limit of 1 RTEPROD sample per establishment per month (maximum of 12 per year) and each establishment is selected for a sample at least two times per year.
The following criteria are used to select an eligible establishment for an RTE product sampling task.
- Each eligible RTE establishment is selected for a sample at least once every 6 months.
- Any eligible establishment with a positive result (either Lm or Salmonella) in the RTEPROD sampling project will be selected for additional RTEPROD sampling tasks in each of the following 6 months.*
- After assigning tasks using the above criteria, the remaining number of sampling tasks each month will be assigned to establishments based on a risk ranking. The risk ranking takes into account:
- The historical percent positive for each product produced at the establishment.
- The daily production volume of each product at the establishment.
- The Listeria alternative used for each product at the establishment or whether the product is not post-lethality exposed.
*NOTE: The additional RTEPROD tasks that are assigned in each of the following 6 months after a positive result are in addition to follow-up sampling of product, food contact, and non-food contact environmental surfaces conducted by Enforcement, Investigation, and Analysis Officers as part of Intensified Verification Testing (IVT) described in FSIS Directive 10,300.1, Intensified Verification Testing Protocol for Sampling of Product, Food Contact Surfaces and Environmental Surfaces for Listeria monocytogenes.
